1Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX
Background, Rationale, and Hypotheses
Primary mediastinal large B-cell lymphoma (PMBL) is an uncommon subtype of large B-cell lymphoma and affects primarily young adults. PMBL has features amenable to target therapy, including CD30 expression and genomic alterations in the programmed T-cell Death-ligand 1 (PD-L1) locus 9p24.1. The trial CheckMate-436, including relapsed/refractory (rr) PMBL patients treated with immune checkpoint inhibitor nivolumab and anti-CD30 antibody-drug conjugated brentuximab vedotin, showed an overall response rate (ORR) of 70-73% and complete response rate (CRR) of 37-43%. Although most PMBL patients cam be cured with frontline standard chemoimmunotherapy (CIT) with or without radiotherapy (XRT), these regimens have early and late toxicities. the outcomee of patients have rr-PMBL is unfavorable. We hypothesize that brentuximab vedotin and nivolumab alone will show ORR in frontline therapy at least as high as in the rr setting for PMBL, brentuximab vedotin and nivolumab used in frontline therapy may avoid evasion of host immune responses and resistance to CIT by tumor cells, brentuximab vedotin and nivolumab could allow deescalating the intensity of the backbone CIT, reduce the number of cycles of CIT for early responders and alleviate the need for consolidative XRT.
Primary mediastinal large B-cell lymphoma (PMBL) is an uncommon subtype of large B-cell lymphoma and affects primarily young adults. PMBL has features amenable to target therapy, including CD30 expression and genomic alterations in the programmed T-cell Death-ligand 1 (PD-L1) locus 9p24.1. The trial CheckMate-436, including relapsed/refractory (rr) PMBL patients treated with immune checkpoint inhibitor nivolumab and anti-CD30 antibody-drug conjugated brentuximab vedotin, showed an overall response rate (ORR) of 70-73% and complete response rate (CRR) of 37-43%. Although most PMBL patients cam be cured with frontline standard chemoimmunotherapy (CIT) with or without radiotherapy (XRT), these regimens have early and late toxicities. the outcomee of patients have rr-PMBL is unfavorable. We hypothesize that brentuximab vedotin and nivolumab alone will show ORR in frontline therapy at least as high as in the rr setting for PMBL, brentuximab vedotin and nivolumab used in frontline therapy may avoid evasion of host immune responses and resistance to CIT by tumor cells, brentuximab vedotin and nivolumab could allow deescalating the intensity of the backbone CIT, reduce the number of cycles of CIT for early responders and alleviate the need for consolidative XRT.
STUDY DESIGN and METHODS
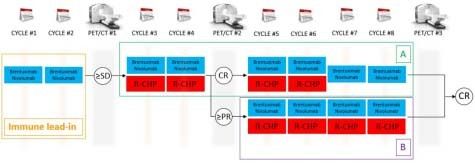
We are conducting a phase II, open-label, single-center clinical trial combining brentuximab vedotin and nivolumab alone and then combined with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for patients with previously untreated PMBL. NCT04745949; PACIFIC: Primary Mediastinal large B-cell lymphoma treated with Antibody therapy, Checkpoint Inhibitor in Frontline with ImmunoChemotherapy. As represented in the figure below, patients will receive brentuximab vedotin 1.8 mg/Kg IV and nivolumab 240 mg flat dose IV day 1 for cycles 1 and 2, in a 21-day cycle of brentuximab vedotin and nivolumab. During cycles 3 and 4, R-CHP (standard dose similar to R-CHOP; vincristine is omitted to avoid cumulative neurotoxicity with brentuximab vedotin) will be added to brentuximab vedotin and nivolumab. Patients who will have achieved complete response (CR) at PET/CT before cycle 5 will receive 2 more cycles of brentuximab vedotin and nivolumab + R-CHP (cycle 5 and 6) and brentuximab vedotin and nivolumab only for cycles 7 and 8 (group A). In case of CR on PET/CT after cycle 8, therapy will be considered completed. If stable disease or progressive disease on PET/CT after cycle 4, patients will be taken off the trial. Patients in partial response (PR) on PET/CT before cycle 5 will receive 4 more cycles of brentuximab vedotin and nivolumab + R-CHP (cycles 5-8; group B). Consolidative XRT is not allowed per protocol. Considering a very high response rate of R-CHOP, this study design will allow for evaluation of the efficacy of frontline brentuximab vedotin and nivolumab without CIT for up to 2 cycles
We are conducting a phase II, open-label, single-center clinical trial combining brentuximab vedotin and nivolumab alone and then combined with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for patients with previously untreated PMBL. NCT04745949; PACIFIC: Primary Mediastinal large B-cell lymphoma treated with Antibody therapy, Checkpoint Inhibitor in Frontline with ImmunoChemotherapy. As represented in the figure below, patients will receive brentuximab vedotin 1.8 mg/Kg IV and nivolumab 240 mg flat dose IV day 1 for cycles 1 and 2, in a 21-day cycle of brentuximab vedotin and nivolumab. During cycles 3 and 4, R-CHP (standard dose similar to R-CHOP; vincristine is omitted to avoid cumulative neurotoxicity with brentuximab vedotin) will be added to brentuximab vedotin and nivolumab. Patients who will have achieved complete response (CR) at PET/CT before cycle 5 will receive 2 more cycles of brentuximab vedotin and nivolumab + R-CHP (cycle 5 and 6) and brentuximab vedotin and nivolumab only for cycles 7 and 8 (group A). In case of CR on PET/CT after cycle 8, therapy will be considered completed. If stable disease or progressive disease on PET/CT after cycle 4, patients will be taken off the trial. Patients in partial response (PR) on PET/CT before cycle 5 will receive 4 more cycles of brentuximab vedotin and nivolumab + R-CHP (cycles 5-8; group B). Consolidative XRT is not allowed per protocol. Considering a very high response rate of R-CHOP, this study design will allow for evaluation of the efficacy of frontline brentuximab vedotin and nivolumab without CIT for up to 2 cycles
Figure 1
KEY ELIGIBILITY CRITERIA
≥18 years with previously untreated PMBL with CD30 expression of ≥1% by immunohistochemistry, stage I (≥ 5cm) to stage IV disease are eligible; Patients with urgent need for cytoreductive treatment, and neuropathy (Grades 2 or Grade 1 with pain) will be excluded.
≥18 years with previously untreated PMBL with CD30 expression of ≥1% by immunohistochemistry, stage I (≥ 5cm) to stage IV disease are eligible; Patients with urgent need for cytoreductive treatment, and neuropathy (Grades 2 or Grade 1 with pain) will be excluded.
ENDPOINTS
Primary endpoint: CR rate (CRR) at the end of therapy (EOT). The maximum sample size for the PMBL cohort is 40 patients, with a target CRR at EOT of 70%. Secondary endpoints: response rate of brentuximab vedotin and nivolumab at the end of the immune lead-in, landmark survival outcomes, and the safety of the combination. The Health-Related QOL EORTC QLQ-C30 instruments will be utilized to evaluate the QOL
Primary endpoint: CR rate (CRR) at the end of therapy (EOT). The maximum sample size for the PMBL cohort is 40 patients, with a target CRR at EOT of 70%. Secondary endpoints: response rate of brentuximab vedotin and nivolumab at the end of the immune lead-in, landmark survival outcomes, and the safety of the combination. The Health-Related QOL EORTC QLQ-C30 instruments will be utilized to evaluate the QOL
TRIAL STATUS
The trial was activated in May 2021 and is actively enrolling at MD Anderson Cancer Center.
The trial was activated in May 2021 and is actively enrolling at MD Anderson Cancer Center.
Figure 2
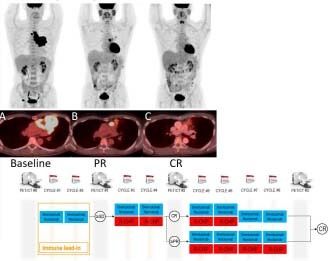
(A) PET-CT images of a 58-year old man with PMBL shows intensely hypermetabolic bulky mediastinal mass before therapy. (B) After 2 cycles of brentuximab vedotin and nivolumab, there was partial response (PR; Lugano classification) marked with anatomic and metabolic improvement of the disease. (C) After 2 additional cycles of brentuximab vedotin, nivolumab + R-CHP, with complete response (CR; Lugano classification).
CONCLUSION
The PACIFIC trial is the first to evaluate brentuximab vedotin and nivolumab and then combined with R-CHP in untreated PMBL. Strong clinical rational with the CheckMate-436 trial to target CD30 and PD-1 in the frontline setting to decrease chemoresistance. Potential future cohorts with adolescents and gray-zone lymphoma patients.
The PACIFIC trial is the first to evaluate brentuximab vedotin and nivolumab and then combined with R-CHP in untreated PMBL. Strong clinical rational with the CheckMate-436 trial to target CD30 and PD-1 in the frontline setting to decrease chemoresistance. Potential future cohorts with adolescents and gray-zone lymphoma patients.
REFERENCES
- Zinzani et al. JCO 2019 CheckMate-436 trial
- Hayden et al. Blood 2020
- Crombie et al. Blood adv 2021
The data in this poster was presented at ASH 2021. Published with permission from the Copyright owner.
