1The University of Texas MD Anderson Cancer Center, Houston, TX, USA;
BACKGROUND
• Progression of disease within 24 months after initial diagnosis (POD24) is an indicator of poor outcomes for patients with mantle cell lymphoma (MCL)1
– In a retrospective analysis of patients with MCL and a median follow-up of 30 months from time of progression, the median overall survival (OS) from time of progression was 12 months for those with POD24, compared with not reached for those without POD24
• KTE-X19, an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, is approved in the United States and European Union for the treatment of relapsed/refractory (R/R) MCL2,3
•The pivotal Phase 2 ZUMA-2 study evaluated KTE-X19 in patients with MCL who were R/R to 1–5 prior therapies, including a Bruton tyrosine kinase inhibitor4
– After a median follow-up of 17.5 months in ZUMA-2 (N=60), the objective response rate (ORR) was 92%, with a complete response (CR) rate of 67%5
• Progression of disease within 24 months after initial diagnosis (POD24) is an indicator of poor outcomes for patients with mantle cell lymphoma (MCL)1
– In a retrospective analysis of patients with MCL and a median follow-up of 30 months from time of progression, the median overall survival (OS) from time of progression was 12 months for those with POD24, compared with not reached for those without POD24
• KTE-X19, an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, is approved in the United States and European Union for the treatment of relapsed/refractory (R/R) MCL2,3
•The pivotal Phase 2 ZUMA-2 study evaluated KTE-X19 in patients with MCL who were R/R to 1–5 prior therapies, including a Bruton tyrosine kinase inhibitor4
– After a median follow-up of 17.5 months in ZUMA-2 (N=60), the objective response rate (ORR) was 92%, with a complete response (CR) rate of 67%5
OBJECTIVE
- To report safety and efficacy outcomes and the pharmacokinetic profile among patients in ZUMA-2 with and without POD24
METHODS
Figure 1. ZUMA-2 Study Design
*Administered after leukapheresis and completed ≥5 days before initiating conditioning chemotherapy; PET-CT was required postbridging. bBone marrow biopsy was to be done at screening, and if positive, not done, or if indeterminate, a biopsy was needed to confirm CR.AE, adverse event; CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; IV, intravenous; MCL, mantle cell lymphoma; ORR, objective response rate; PO, oral; R/R, relapsed/refractory.
• Product attributes, CAR T-cell levels in blood, and cytokine levels in serum were analyzed using previously described methods6
• Safety outcomes, pharmacological profile, and product attributes are reported for all 68 patients treated with KTE-X19 (2×106 cells/kg)
• Efficacy outcomes are reported in the 60 treated patients with ≥1 year of follow-up (median 17.5 months)–Minimal residual disease (MRD) was assessed in patients with available samples at Week 4 with a sensitivity of 1 in 100,000 cells
• Data are presented with the data cutoff date of December 31, 2019
Figure 1. ZUMA-2 Study Design
*Administered after leukapheresis and completed ≥5 days before initiating conditioning chemotherapy; PET-CT was required postbridging. bBone marrow biopsy was to be done at screening, and if positive, not done, or if indeterminate, a biopsy was needed to confirm CR.AE, adverse event; CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; IV, intravenous; MCL, mantle cell lymphoma; ORR, objective response rate; PO, oral; R/R, relapsed/refractory.
• Product attributes, CAR T-cell levels in blood, and cytokine levels in serum were analyzed using previously described methods6
• Safety outcomes, pharmacological profile, and product attributes are reported for all 68 patients treated with KTE-X19 (2×106 cells/kg)
• Efficacy outcomes are reported in the 60 treated patients with ≥1 year of follow-up (median 17.5 months)–Minimal residual disease (MRD) was assessed in patients with available samples at Week 4 with a sensitivity of 1 in 100,000 cells
• Data are presented with the data cutoff date of December 31, 2019
Figure 1
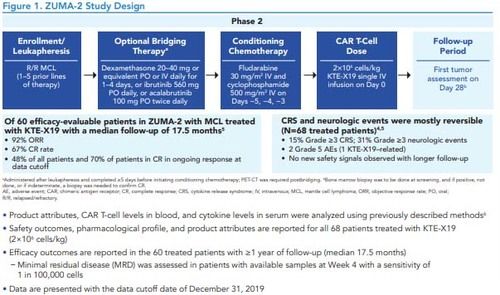
Table 1
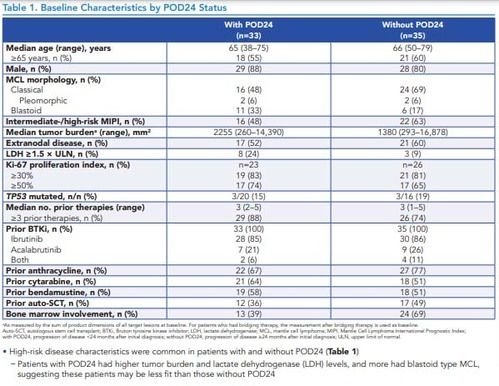
Table 2
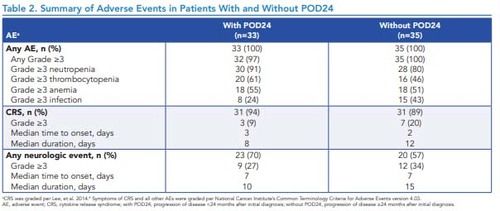
Table 3
RESULTS
Table 1. Baseline Characteristics by POD24 Status
*As measured by the sum of product dimensions of all target lesions at baseline. For patients who had bridging therapy, the measurement after bridging therapy is used as baseline.Auto-SCT, autologous stem cell transplant; BTKi, Bruton tyrosine kinase inhibitor; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MIPI, Mantle Cell Lymphoma International Prognostic Index; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis; ULN, upper limit of normal.
Figure 2. ORR by IRRC Assessment in Patients With and Without POD24
*With POD24 (n=28)PDORRSDWithout POD24 (n=32)aPDBest Response, %93% (n=26)61% CR(n=17)32% PR(n=9)72% CR(n=23)19% PR(n=6)4%(n=1)4%(n=1)3%(n=1)3%(n=1)91% (n=29)Assessed by an IRRC according to the Lugano Classification.7aOne patient was not evaluable.CR, complete response; IRRC, Independent Radiology Review Committee; ORR, objective response rate; PD, progressive disease; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis; PR, partial response; SD, stable disease
• The ORR was similar among patients with and without POD24, with a slightly higher CR rate in patients without POD24 (Figure 2)
• Similar rates of MRD-negativity were also observed among patients with (82%; n=9/11) and without (79%; n=15/19) POD24
Figure 3. Duration of Response, Progression-Free Survival, and Overall Survival by POD24 Status
*Of responding patients.NE, not estimable; NR, not reached; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
• Median progression-free survival (PFS) was 11.3 months (95% CI, 6.0–not estimable [NE]) in patients with POD24 and was 29.3 months (95% CI, 14.5–NE) in patients without POD24 (Figure 3)
• Medians for duration of response (DOR) and OS were not reached in either group (Figure 3)
• Among all enrolled patients (N=74), median OS was not reached in patients with and without POD24; estimated 12-month OS rates were 72% and 81%, respectively
Table 1. Baseline Characteristics by POD24 Status
*As measured by the sum of product dimensions of all target lesions at baseline. For patients who had bridging therapy, the measurement after bridging therapy is used as baseline.Auto-SCT, autologous stem cell transplant; BTKi, Bruton tyrosine kinase inhibitor; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MIPI, Mantle Cell Lymphoma International Prognostic Index; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis; ULN, upper limit of normal.
- High-risk disease characteristics were common in patients with and without POD24 (Table 1)–Patients with POD24 had higher tumor burden and lactate dehydrogenase (LDH) levels, and more had blastoid type MCL, suggesting these patients may be less fit than those without POD24
Figure 2. ORR by IRRC Assessment in Patients With and Without POD24
*With POD24 (n=28)PDORRSDWithout POD24 (n=32)aPDBest Response, %93% (n=26)61% CR(n=17)32% PR(n=9)72% CR(n=23)19% PR(n=6)4%(n=1)4%(n=1)3%(n=1)3%(n=1)91% (n=29)Assessed by an IRRC according to the Lugano Classification.7aOne patient was not evaluable.CR, complete response; IRRC, Independent Radiology Review Committee; ORR, objective response rate; PD, progressive disease; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis; PR, partial response; SD, stable disease
• The ORR was similar among patients with and without POD24, with a slightly higher CR rate in patients without POD24 (Figure 2)
• Similar rates of MRD-negativity were also observed among patients with (82%; n=9/11) and without (79%; n=15/19) POD24
Figure 3. Duration of Response, Progression-Free Survival, and Overall Survival by POD24 Status
*Of responding patients.NE, not estimable; NR, not reached; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
• Median progression-free survival (PFS) was 11.3 months (95% CI, 6.0–not estimable [NE]) in patients with POD24 and was 29.3 months (95% CI, 14.5–NE) in patients without POD24 (Figure 3)
• Medians for duration of response (DOR) and OS were not reached in either group (Figure 3)
• Among all enrolled patients (N=74), median OS was not reached in patients with and without POD24; estimated 12-month OS rates were 72% and 81%, respectively
Figure 2
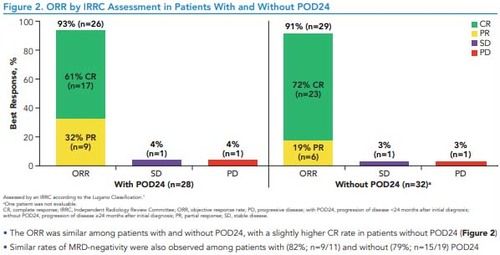
Figure 3
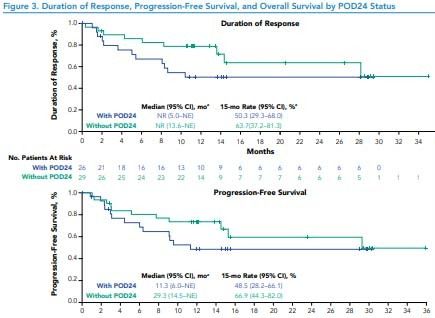
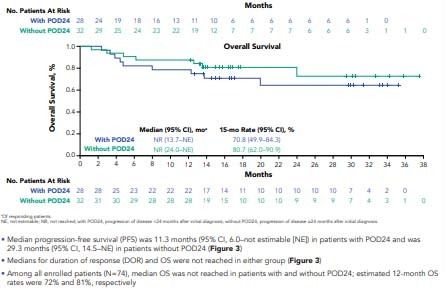
Figure 4
RESULTS (CONTINUED)
Table 2. Summary of Adverse Events in Patients With and Without POD24
• Incidences of Grade ≥3 adverse events were generally similar in patients with and without POD24 (Table 2).
- Incidence of thrombocytopenia and neutropenia appeared higher in patients with POD24 than those without POD24
–Incidences of infection appeared lower among patients with POD24 than those without POD24
• There were no cases of Grade 5 cytokine release syndrome, KTE-X19–related secondary malignancies, or replication-competent retrovirus in either group
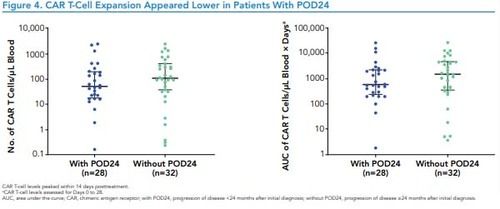
Figure 4. CAR T-Cell Expansion Appeared Lower in Patients With POD24
*CAR T-cell levels peaked within 14 days posttreatment.
* CAR T-cell levels assessed for Days 0 to 28.
AUC, area under the curve; CAR, chimeric antigen receptor; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis
• In patients with POD24, median peak CAR T-cell levels and median area under the curve (AUC) were 53.4 cells/μL(range, 0.2–2566.0) and 583.4 cells/μL × days (range, 1.8–27,743.6; Figure 4)
–Patients without POD24 had median peak CAR T-cell levels and median AUC of 112.4 cells/μL (range, 0.2–2589.0) and1588.3 cells/μL × days (range, 3.8–27,238.7
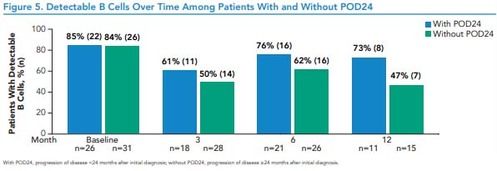
Figure 5. Detectable B Cells Over Time Among Patients With and Without POD24
*With POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
• Among efficacy-evaluable patients with available data, B cells were detectable by 12 months in 8/11 (73%) patients with POD24 and 7/15 patients (47%) without POD24 (Figure 5)
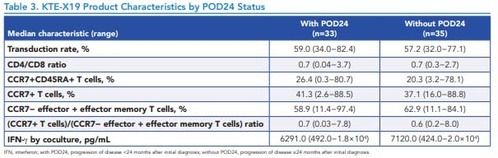
Table 3. KTE-X19 Product Characteristics by POD24 Status
*IFN, interferon; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
Table 2. Summary of Adverse Events in Patients With and Without POD24
• Incidences of Grade ≥3 adverse events were generally similar in patients with and without POD24 (Table 2).
- Incidence of thrombocytopenia and neutropenia appeared higher in patients with POD24 than those without POD24
–Incidences of infection appeared lower among patients with POD24 than those without POD24
• There were no cases of Grade 5 cytokine release syndrome, KTE-X19–related secondary malignancies, or replication-competent retrovirus in either group
Figure 4. CAR T-Cell Expansion Appeared Lower in Patients With POD24
*CAR T-cell levels peaked within 14 days posttreatment.
* CAR T-cell levels assessed for Days 0 to 28.
AUC, area under the curve; CAR, chimeric antigen receptor; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis
• In patients with POD24, median peak CAR T-cell levels and median area under the curve (AUC) were 53.4 cells/μL(range, 0.2–2566.0) and 583.4 cells/μL × days (range, 1.8–27,743.6; Figure 4)
–Patients without POD24 had median peak CAR T-cell levels and median AUC of 112.4 cells/μL (range, 0.2–2589.0) and1588.3 cells/μL × days (range, 3.8–27,238.7
Figure 5. Detectable B Cells Over Time Among Patients With and Without POD24
*With POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
• Among efficacy-evaluable patients with available data, B cells were detectable by 12 months in 8/11 (73%) patients with POD24 and 7/15 patients (47%) without POD24 (Figure 5)
Table 3. KTE-X19 Product Characteristics by POD24 Status
*IFN, interferon; with POD24, progression of disease <24 months after initial diagnosis; without POD24, progression of disease ≥24 months after initial diagnosis.
- KTE-X19 product characteristics were similar among patients with and without POD24 (Table 3)
CONCLUSION
• After a median of 17.5 months of follow-up, KTE-X19 provided a high CR rate in patients with and without POD24, with median DOR and OS not reached in either group
–Median PFS appeared to be shorter among patients with POD24, compared with those without POD24
• At baseline, patients with POD24 were more likely to have high-risk disease characteristics (high tumor burden, high LDH levels, and blastoid MCL) than those without POD24
• Safety profiles and product characteristics of patients with and without POD24 were generally similar
• Patients with POD24 appeared to have lower CAR T-cell expansion than those without POD24
• Earlier intervention with CD19-directed CAR T-cell therapy may benefit patients with MCL with known high-risk factors1
• After a median of 17.5 months of follow-up, KTE-X19 provided a high CR rate in patients with and without POD24, with median DOR and OS not reached in either group
–Median PFS appeared to be shorter among patients with POD24, compared with those without POD24
• At baseline, patients with POD24 were more likely to have high-risk disease characteristics (high tumor burden, high LDH levels, and blastoid MCL) than those without POD24
• Safety profiles and product characteristics of patients with and without POD24 were generally similar
• Patients with POD24 appeared to have lower CAR T-cell expansion than those without POD24
• Earlier intervention with CD19-directed CAR T-cell therapy may benefit patients with MCL with known high-risk factors1
REFERENCES
1. Visco C, et al. Br J Haematol. 2019;185:940-944.
2. TECARTUS® (brexucabtagene autoleucel) Prescribing information. Kite Pharma, Inc; 2021.
3. TECARTUS® (autologous anti-CD19-transduced CD3+ cells) Summary of product characteristics. Kite Pharma EU B.V.; 2021.
4. Wang M, et al. N Engl J Med. 2020;382:1331-1342.
5. Wang M, et al. Blood. 2020; 136(Suppl 1):20-22.
6. Locke FL, et al. Mol Ther. 2017;25:285-295.
7. Cheson BD, et al. J Clin Oncol. 2014;32:3059-3068.
8. Lee DW, et al. Blood. 2014;124:188-195
1. Visco C, et al. Br J Haematol. 2019;185:940-944.
2. TECARTUS® (brexucabtagene autoleucel) Prescribing information. Kite Pharma, Inc; 2021.
3. TECARTUS® (autologous anti-CD19-transduced CD3+ cells) Summary of product characteristics. Kite Pharma EU B.V.; 2021.
4. Wang M, et al. N Engl J Med. 2020;382:1331-1342.
5. Wang M, et al. Blood. 2020; 136(Suppl 1):20-22.
6. Locke FL, et al. Mol Ther. 2017;25:285-295.
7. Cheson BD, et al. J Clin Oncol. 2014;32:3059-3068.
8. Lee DW, et al. Blood. 2014;124:188-195
ACKNOWLEDGMENTS
• The patients, families, friends, and caregivers
• The study investigators, coordinators, and health care staff at each study site
• The authors would like to thank Rubina Siddiqi, PhD, of Kite, a Gilead Company, for her expertise and strategic contributions
• Medical writing support was provided by Danielle Luebke, PhD, of Nexus Global Group Science, funded by Kite, a Gilead Company
• This study was funded by Kite, a Gilead Compan
• The patients, families, friends, and caregivers
• The study investigators, coordinators, and health care staff at each study site
• The authors would like to thank Rubina Siddiqi, PhD, of Kite, a Gilead Company, for her expertise and strategic contributions
• Medical writing support was provided by Danielle Luebke, PhD, of Nexus Global Group Science, funded by Kite, a Gilead Company
• This study was funded by Kite, a Gilead Compan
Figure 5
DISCLOSURES
MLW: honoraria from Janssen, OMI, DAVA Oncology, and PeerView Institute for Medical Education; consultancy or advisory role for Kite, a Gilead Company, Celgene, Juno, Janssen, Pharmacyclics, AstraZeneca, MORE Health, Pulse Biosciences, InnoCare, Loxo Oncology, CStone, and VelosBio; research funding from Kite, a Gilead Company, Janssen, AstraZeneca, Acerta, Juno, BeiGene, Celgene, BioInvent, Oncternal, Loxo Oncology, VelosBio, Molecular Templates, InnoCare, and Lilly; and travel support from Kite, a Gilead Company, Janssen, AstraZeneca, DAVA Oncology, OMI, and Pharmacyclics. JM: honoraria from Kyowa Kirin and Seattle Genetics; consultancy or advisory role for Pharmacyclics, Bayer, Kite, a Gilead Company, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa Kirin, Alexion, Fosun Kite, Innovent, Seattle Genetics, and BeiGene; speakers’ bureau participation for Kite, a Gilead Company, Kyowa Kirin, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Juno/Celgene/Bristol Myers Squibb, Genentech/Roche, and AbbVie; research funding from Bayer, Kite, a Gilead Company, Celgene, Merck, Portola Pharmaceuticals, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, and Millennium. AG: employment with Regional Cancer Care Associates/OMI; leadership role at COTA (Cancer Outcome Tracking Analysis) and Genomic Testing Cooperative; stock or other ownership in COTA and Genomic Testing Cooperative; honoraria from Celgene, Elsevier PracticeUpdate: Oncology, Kite, a Gilead Company, AstraZenca, Xcenda, OncLive Peer Exchange, Janssen, Novartis, MorphoSys, Incyte, and Pharmacyclics; consultancy or advisory role for Physcians' Education Resource, Celgene, Elsevier PracticeUpdate: Oncology, Janssen, Kite, a Gilead Company, Medscape, Michael J. Hennessy Associates, Inc., Novartis, and Pharmacyclics; research funding from Acerta, AstraZeneca, Celgene, Genentech, Hoffmann-La Roche, Infinity Pharmaceuticals, Janssen, Karyopharm, and Pharmacyclics; and other relationships with MorphoSys, Incyte Steering Committee, and AstraZeneca MCL Steering Committee. FLL: consultancy or advisory role for Kite, a Gilead Company, Novartis, Amgen, Celgene/Bristol Myers Squibb, GammaDelta Therapeutics, Iovance, Bluebird Bio, Wugen Inc., Calibr, Cellular Biomedicine Group Inc., and Allogen; and research support from Kite, a Gilead Company. CAJ: honoraria from Kite, a Gilead Company, Bristol Myers Squibb, Celgene, Novartis, Humanigen, Precision BioSciences, Bluebird Bio, Nkarta, Lonza, and AbbVie; consultancy or advisory role for Kite, a Gilead Company, Celgene, Novartis, Bristol Myers Squibb, Precision BioSciences, Nkarta, Lonza, Pfizer, Humanigen, AbbVie, and Bluebird Bio; speakers' bureau participation for Axis and Clinical Care Options; research funding from Kite, a Gilead Company, and Pfizer; and travel support from Kite, a Gilead Company, Celgene, Novartis, Bristol Myers Squibb, Precision Biosciences, Lonza, Pfizer, and Humanigen. BTH:honoraria from Kite, a Gilead Company; consulting or advisory role with Kite, a Gilead Company; research funding from Kite, a Gilead Company; travel, accommodations, expenses from Kite, a Gilead Company. JMT: stock or other ownership in Genmab, Corvus, Marker Therapeutics, and Bluebird Bio; consulting or advisory role with Kite, a Gilead Company, Celgene, Immune Design, and Celldex Therapeutics; research funding from Bristol Myers Squibb, Kite, a Gilead Company, Spectrum Pharmaceuticals, and Merck; travel, accommodations, expenses from Bristol Myers Squibb, and Kite, a Gilead Company. HH: consultancy or advisory role for Kite, a Gilead Company, Bayer, Rigel, Johnson & Johnson, and Bristol Myers Squibb; speakers' bureau participation for Kite, a Gilead Company, Seattle Genetics, and Rigel; research funding from Kite, a Gilead Company, Unum, Bristol Myers Squibb, Novartis, Roche, ADC Therapeutics, and Seattle Genetics; patents, royalties, or other intellectual property from a pending patent. SJ: consultancy or advisory role for Kite, a Gilead Company, Juno, Novartis, and CRISPR; research funding from Kite, a Gilead Company, and Novartis. IWF: employment with Sarah Cannon Research Institute; stock or other ownership in Johnson & Johnson; consultancy or advisory role for AbbVie, AstraZeneca, BeiGene, Gilead, Great Point Partners, Iksuda Therapeutics, Janssen, Juno, Kite, a Gilead Company, MorphoSys, Nurix Therapeutics, Pharmacyclics, Roche, Seattle Genetics, Takeda, Unum Therapeutics (Cogent Biosciences), Verastem, and Yingli Pharmaceuticals; and research funding from AbbVie, Acerta, Agios, ArQule, AstraZeneca, BeiGene, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Curis, F. Hoffmann-La Roche, Forma, Forty Seven, Genentech, Gilead, IGM, Incyte, Infinity Pharmaceuticals, Janssen, Juno, Karyopharm, Kite, a Gilead Company, Loxo Oncology, Merck, MorphoSys, Novartis, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Roche, Seattle Genetics, Takeda, Teva, TG Therapeutics, Trillium Therapeutics, Triphase Research & Development Corp., Unum Therapeutics (Cogent Biosciences), and Verastem. PAM: employment with Colorado Blood Cancer Institute Medical Group; consulting or advisory role with Kite, a Gilead Company; speakers' bureau with Kite, a Gilead Company; research funding from Kite, a Gilead Company. DBM: consultancy or advisory role for Kite, a Gilead Company, Novartis, Juno/Celgene/Bristol Myers Squibb, Adaptive Biotechnologies, Pharmacyclics, Janssen, Allogene, Precision BioSciences, Adicet Bio, Takeda, and Miltenyi; research funding from Kite, a Gilead Company, Novartis, Juno/Celgene/Bristol Myers Squibb, Adaptive Biotechnologies, Pharmacyclics, Allogene, Precision BioSciences, and Adicet; and patents, royalties, or other intellectual property from Pharmacyclics. MJK: honoraria from Kite, a Gilead Company, Novartis, and Miltenyi; consultancy or advisory role for Kite, a Gilead Company, Novartis, and Miltenyi; research funding from Roche, Takeda, and Celgene; travel support from Kite, a Gilead Company, Novartis, and Miltenyi. KB: honoraria from Kite, a Gilead Company, Takeda, Roche, and Celgene; consultancy or advisory role for Kite, a Gilead Company, Takeda, Roche, and Sandoz; and travel support from Roche. MST: consultancy or advisory role for Amgen, Kite, a Gilead Company, Celgene, Roche, and Regeneron; and research funding from Amgen, Kite, a Gilead Company, Roche, MacroGenics, and Regeneron. XF, IK, and RS: employment with Kite, a Gilead Company; stock or other ownership in Gilead Sciences. WP: consultancy or advisory role for Kite, a Gilead Company, and Curis; and research funding from Seattle Genetics and Genentech.
The data in this poster was presented at ASCO 2021. Published with permission from the Copyright owner.
