ABSTRACT
OBJECTIVE:
10-Year survival (10YS) after radical surgery for gastric cancer (GC) patients (GCP) (T1-4N0-2M0) was analyzed
METHODS:
We analyzed data of 796 consecutive GCP (age=57.1±9.4 years; tumor size=5.4±3.1 cm) radically operated (R0) and monitored in 1975-2021 (m=556, f=240; distal gastrectomies-G=461, proximal G=165, total G=170, D2 lymph node dissection=551; combined G with resection of 1-7 adjacent organs (pancreas, liver, diaphragm, esophagus, colon transversum, splenectomy, small intestine, kidney, adrenal gland, etc.)=245; D3-4 lymph node dissection=245; only surgery-S=623, adjuvant chemoimmunotherapy-AT=173: 5FU+thymalin/taktivin; T1=237, T2=220, T3=182, T4=157; N0=435, N1=109, N2=252, M0=796; G1=222, G2=164, G3=410; early GC=164, invasive GC=632; Variables selected for 10YS study were input levels of 45 blood parameters, sex, age, TNMG, cell type, tumor size. Survival curves were estimated by the Kaplan-Meier method. Differences in curves between groups of GCP were evaluated using a log-rank test. Multivariate Cox modeling, discriminant analysis, clustering, SEPATH, Monte Carlo, bootstrap and neural networks computing were used to determine any significant dependence.
RESULTS:
Overall life span (LS) was 2130.8±2304.3 days and cumulative 5-year survival (5YS) reached 58.4%, 10 years – 52.4%, 20 years – 40.4%. 316 GCP lived more than 5 years (LS=4316.1±2292.9 days), 169 GCP – more than 10 years (LS=5919.5±2020 days). 294 GCP died because of GC (LS=640.6±347.1 days). AT significantly improved 10YS (62.3% vs. 50.5%) (P=0.0228 by log-rank test) for GCP. Cox modeling displayed that 10YS of LCP significantly depended on: phase transition (PT) early-invasive GC in terms of synergetics, PT N0—N12, cell ratio factors (ratio between cancer cells- CC and blood cells subpopulations), G1-3, AT, blood cell circuit, prothrombin index, hemorrhage time, residual nitrogen, age, sex, procedure type (P=0.000-0.039). Neural networks, genetic algorithm selection and bootstrap simulation revealed relationships between 10YS and healthy cells/CC (rank=1), PT early-invasive GC (rank=2), PT N0—N12(rank=3), erythrocytes/CC (4), thrombocytes/CC (5), monocytes/CC (6), segmented neutrophils/CC (7), eosinophils/CC (8), leucocytes/CC (9), lymphocytes/CC (10), stick neutrophils/CC (11). Correct prediction of 5YS was 100% by neural networks computing (area under ROC curve=1.0; error=0.0).
CONCLUSIONS:
10-Year survival of GCP after radical procedures significantly depended on: 1) PT early-invasive cancer; 2) PT N0--N12; 3) cell ratio factors; 4) blood cell circuit; 5) biochemical factors; 6) hemostasis system; 7) AT; 8) GC characteristics; 9) anthropometric data; 10) surgery type. Optimal diagnosis and treatment strategies for GC are: 1) screening and early detection of GC; 2) availability of experienced abdominal surgeons because of complexity of radical procedures; 3) aggressive en block surgery and adequate lymph node dissection for completeness; 4) precise prediction; 5) adjuvant chemoimmunotherapy for GCP with unfavorable prognosis.
10-Year survival (10YS) after radical surgery for gastric cancer (GC) patients (GCP) (T1-4N0-2M0) was analyzed
METHODS:
We analyzed data of 796 consecutive GCP (age=57.1±9.4 years; tumor size=5.4±3.1 cm) radically operated (R0) and monitored in 1975-2021 (m=556, f=240; distal gastrectomies-G=461, proximal G=165, total G=170, D2 lymph node dissection=551; combined G with resection of 1-7 adjacent organs (pancreas, liver, diaphragm, esophagus, colon transversum, splenectomy, small intestine, kidney, adrenal gland, etc.)=245; D3-4 lymph node dissection=245; only surgery-S=623, adjuvant chemoimmunotherapy-AT=173: 5FU+thymalin/taktivin; T1=237, T2=220, T3=182, T4=157; N0=435, N1=109, N2=252, M0=796; G1=222, G2=164, G3=410; early GC=164, invasive GC=632; Variables selected for 10YS study were input levels of 45 blood parameters, sex, age, TNMG, cell type, tumor size. Survival curves were estimated by the Kaplan-Meier method. Differences in curves between groups of GCP were evaluated using a log-rank test. Multivariate Cox modeling, discriminant analysis, clustering, SEPATH, Monte Carlo, bootstrap and neural networks computing were used to determine any significant dependence.
RESULTS:
Overall life span (LS) was 2130.8±2304.3 days and cumulative 5-year survival (5YS) reached 58.4%, 10 years – 52.4%, 20 years – 40.4%. 316 GCP lived more than 5 years (LS=4316.1±2292.9 days), 169 GCP – more than 10 years (LS=5919.5±2020 days). 294 GCP died because of GC (LS=640.6±347.1 days). AT significantly improved 10YS (62.3% vs. 50.5%) (P=0.0228 by log-rank test) for GCP. Cox modeling displayed that 10YS of LCP significantly depended on: phase transition (PT) early-invasive GC in terms of synergetics, PT N0—N12, cell ratio factors (ratio between cancer cells- CC and blood cells subpopulations), G1-3, AT, blood cell circuit, prothrombin index, hemorrhage time, residual nitrogen, age, sex, procedure type (P=0.000-0.039). Neural networks, genetic algorithm selection and bootstrap simulation revealed relationships between 10YS and healthy cells/CC (rank=1), PT early-invasive GC (rank=2), PT N0—N12(rank=3), erythrocytes/CC (4), thrombocytes/CC (5), monocytes/CC (6), segmented neutrophils/CC (7), eosinophils/CC (8), leucocytes/CC (9), lymphocytes/CC (10), stick neutrophils/CC (11). Correct prediction of 5YS was 100% by neural networks computing (area under ROC curve=1.0; error=0.0).
CONCLUSIONS:
10-Year survival of GCP after radical procedures significantly depended on: 1) PT early-invasive cancer; 2) PT N0--N12; 3) cell ratio factors; 4) blood cell circuit; 5) biochemical factors; 6) hemostasis system; 7) AT; 8) GC characteristics; 9) anthropometric data; 10) surgery type. Optimal diagnosis and treatment strategies for GC are: 1) screening and early detection of GC; 2) availability of experienced abdominal surgeons because of complexity of radical procedures; 3) aggressive en block surgery and adequate lymph node dissection for completeness; 4) precise prediction; 5) adjuvant chemoimmunotherapy for GCP with unfavorable prognosis.
Introduction
Gastric cancer (GC) is the number two killer in the world in the structure of mortality from malignant neoplasms. Nevertheless, there is practically no analysis of 10-year survival of GC patients (GCP) in the literature. But information on the 10-year survival rate is extremely important in optimizing the treatment and diagnostic process in oncology and especially for extremely aggressive cancer –GC. The high mortality rate associated with GC is primarily due to the high incidence of late stage and the lack of curative management for the majority of GCP. Up to 70-90% of GCP present with stage III-IV disease. The role of adjuvant chemotherapy or chemoimmunotherapy after complete gastrectomies in GCP with stage II-IV remains controversial [1]. Moreover, the optimal treatment plan in general and optimal approach for adjuvant chemotherapy in particular has not been defined and long-term prognosis of GCP especially with stage III-IV remains poor, because of local relapse and distant metastases, with the real 5-year survival rate after radical procedures only 30-35% [2]. One of the approaches developed involves aggressive en-block surgery and complete lymphadenectomy. Another of the modern approaches developed to enhance the efficacy of surgery is the combination of chemotherapy and immunotherapy or gene therapy which offers the advantage of exposing GC cell population for drugs and immune factors thus obviating cancer cell-cycle cytotoxic and host-immunoprotective effects [3-4]. Nevertheless, very few studies have demonstrated convincing clinical results. We developed optimal treatment strategies that incorporate bolus chemotherapy and immunotherapy after radical, aggressive en-block surgery.
PATIENTS AND METHODS
We conducted this study from 1975 to 2021. 796 consecutive GCP (male – 556, female – 240; age=57.1±9.4 years, tumor size=5.4±3.1 cm) (mean±standard deviation) entered this trial. All GCP were white Europeans. Patients were not considered eligible if they had stage IV, previous treatment with chemotherapy, immunotherapy or radiotherapy or if there were two primary tumors of the time of diagnosis. Patients after non-radical procedures, postoperative died GCP were excluded to provide a homogeneous patient group. The preoperative staging protocol included clinical history, physical examination, complete blood count with differentials, biochemistry and electrolyte panel, chest X-rays, roentgenoesophagogastroscopy, abdominal ultrasound, fibroesophagogastroscopy, electrocardiogram. Computed tomography scan of upper abdomen, liver and bone radionucle scan were performed whenever needed. All GCP were diagnosed with histologically confirmed GC. All had measurable tumor and ECOG performance status 0 or 1. Before any treatment each patient was carefully examined by medical panel composed of surgeon and chemotherapeutist to confirm the stage of disease. All patients signed a written informed consent form approved by the local Institutional Review Board.
The initial treatment was started with radical procedures (complete gastrectomies with lesser and major omentum and lymph node dissection). The present analysis was restricted to GCP with complete resected tumors with negative surgical resection margin (R0) and with N1-N2 nodes. Surgical complete resection consisted of total gastrectomy in 170, distal gastrectomy in 461, proximal gastrectomy in 165. Among these, 245 GCP underwent combined and extensive radical procedures with resection of 1-7 adjacent organs (esophagus, duodenum, diaphragm, mesocolon, colon transversum, liver, splenectomy, left hemipancreatectomy, etc.). 551 patients underwent routine lymph nodal D2-dissection. Extensive lymph nodal D3-4-dissection was performed in 245 GCP. All GCP were postoperatively staged according to the TNM-classification. Histological examination showed intestinal adenocarcinoma in 457, diffuse adenocarcinoma - in 320 and mixed adenocarcinoma - in 19 patients. The pathological TNM stage was I in 108, II – in 160, III - in 453 patients; the pathological T stage was T1 in 237, T2 - in 220, T3 - in 182, T4 - in 157 cases; the pathological N stage was N0 in 435, N1 - in 109, N2 - in 252 patients. The tumor differentiation was graded as G1 in 222, G2 - in 164, G3 - in 410 cases. The pathological P stage was P1 in 117, P2 – in 78 P3 – in 142 and P4 – in 459 patients. After surgery postoperative chemoimmunotherapy were accomplished GCP in ECOG performance status 0 or 1.
All patients (408 GCP) were divided between the two protocol treatment: 1) surgery and adjuvant chemoimmunotherapy (173 GCP – group A); 2) surgery alone without any adjuvant treatment (623 GCP – group B) – the control group.
The initial treatment was started with radical procedures (complete gastrectomies with lesser and major omentum and lymph node dissection). The present analysis was restricted to GCP with complete resected tumors with negative surgical resection margin (R0) and with N1-N2 nodes. Surgical complete resection consisted of total gastrectomy in 170, distal gastrectomy in 461, proximal gastrectomy in 165. Among these, 245 GCP underwent combined and extensive radical procedures with resection of 1-7 adjacent organs (esophagus, duodenum, diaphragm, mesocolon, colon transversum, liver, splenectomy, left hemipancreatectomy, etc.). 551 patients underwent routine lymph nodal D2-dissection. Extensive lymph nodal D3-4-dissection was performed in 245 GCP. All GCP were postoperatively staged according to the TNM-classification. Histological examination showed intestinal adenocarcinoma in 457, diffuse adenocarcinoma - in 320 and mixed adenocarcinoma - in 19 patients. The pathological TNM stage was I in 108, II – in 160, III - in 453 patients; the pathological T stage was T1 in 237, T2 - in 220, T3 - in 182, T4 - in 157 cases; the pathological N stage was N0 in 435, N1 - in 109, N2 - in 252 patients. The tumor differentiation was graded as G1 in 222, G2 - in 164, G3 - in 410 cases. The pathological P stage was P1 in 117, P2 – in 78 P3 – in 142 and P4 – in 459 patients. After surgery postoperative chemoimmunotherapy were accomplished GCP in ECOG performance status 0 or 1.
All patients (408 GCP) were divided between the two protocol treatment: 1) surgery and adjuvant chemoimmunotherapy (173 GCP – group A); 2) surgery alone without any adjuvant treatment (623 GCP – group B) – the control group.
All 173 patients completed adjuvant chemoimmunotherapy (group A): 1 cycle of bolus chemotherapy was initiated 10-14 days after complete resections and consisted of fluorouracil (5-FU) 500 mg/m2 intravenously (IV) for 5 days. Immunotherapy consisted thymalin or taktivin 20 mg intramuscularly on days 1, 2, 3, 4 and 5. These immunomodulators produced by Pharmaceutics of Russian Federation (Novosibirsk) and approved by Ministry of Health of Russian Federation. Thymalin and taktivin are preparations from calf thymus, which stimulate proliferation of blood T-cell and B-cell subpopulations and their response [10]. The importance must be stressed of using immunotherapy in combination with chemotherapy, because immune dysfunctions of the cell-mediated and humoral response were induced by tumor, surgical trauma and chemotherapy [6]. Such immune deficiency induced generalization of GC and compromised the long-term therapeutic result. In this sense immunotherapy shielded human organism from side and adverse effects of basic treatment. 4-5 courses of adjuvant chemoimmunotherapy were repeated every 28-day. During chemoimmunotherapy antiemetics were administered. Gastrointestinal side effects, particularly nausea and vomiting, were mild, and chemoimmunotherapy was generally well tolerated. Severe leukopenia, neutropenia, anemia and trombocytopenia occurred infrequently. There were no treatment-related deaths.
A follow-up examination was, generally, done every 3 month for the first 2 years, every 6 month after that and yearly after 5 years, including a physical examination, a complete blood count, blood chemistry, chest roentgenography. Endoscopy and abdominal ultrasound were done every 6-month for the first 3 years and yearly after that. Zero time was the data of surgical procedures. No one was lost during the follow-up period and we regarded the outcome as death through personal knowledge, physician's reports, autopsy or death certificates. Survival time (days) was measured from the date of surgery until death or the most-recent date of follow-up for surviving patients.
Variables selected for 10-year survival and life span study were sex, age, TNM, cell type, tumor size. Survival curves were estimated by the Kaplan-Meier method. Differences in curves between groups of GCP were evaluated using a log-rank test. Multivariate proportional hazard Cox regression, structural equation modeling (SEPATH), Monte Carlo simulation and neural networks computing were used to determine any significant dependence [11-15]. Neural networks computing, system, biometric and statistical analyses were conducted using CLASS-MASTER program (Stat Dialog, Inc., Moscow, Russia), SANI program (Stat Dialog, Inc., Moscow, Russia), STATISTICA and STATISTICA Neural Networks program (Stat Soft, Inc., Tulsa, OK, the USA), DEDUCTOR program (BaseGroup Labs, Inc., Riazan, Russia), SPSS (SPSS Inc., Chicago, IL, USA), Table Curve3D (Systat Software Inc., San Hose, CA, USA). All tests were considered significant when the resulting P value was less than 0.05.
A follow-up examination was, generally, done every 3 month for the first 2 years, every 6 month after that and yearly after 5 years, including a physical examination, a complete blood count, blood chemistry, chest roentgenography. Endoscopy and abdominal ultrasound were done every 6-month for the first 3 years and yearly after that. Zero time was the data of surgical procedures. No one was lost during the follow-up period and we regarded the outcome as death through personal knowledge, physician's reports, autopsy or death certificates. Survival time (days) was measured from the date of surgery until death or the most-recent date of follow-up for surviving patients.
Variables selected for 10-year survival and life span study were sex, age, TNM, cell type, tumor size. Survival curves were estimated by the Kaplan-Meier method. Differences in curves between groups of GCP were evaluated using a log-rank test. Multivariate proportional hazard Cox regression, structural equation modeling (SEPATH), Monte Carlo simulation and neural networks computing were used to determine any significant dependence [11-15]. Neural networks computing, system, biometric and statistical analyses were conducted using CLASS-MASTER program (Stat Dialog, Inc., Moscow, Russia), SANI program (Stat Dialog, Inc., Moscow, Russia), STATISTICA and STATISTICA Neural Networks program (Stat Soft, Inc., Tulsa, OK, the USA), DEDUCTOR program (BaseGroup Labs, Inc., Riazan, Russia), SPSS (SPSS Inc., Chicago, IL, USA), Table Curve3D (Systat Software Inc., San Hose, CA, USA). All tests were considered significant when the resulting P value was less than 0.05.
FigureS 1-4
Click on each figure to enlarge
RESULTS
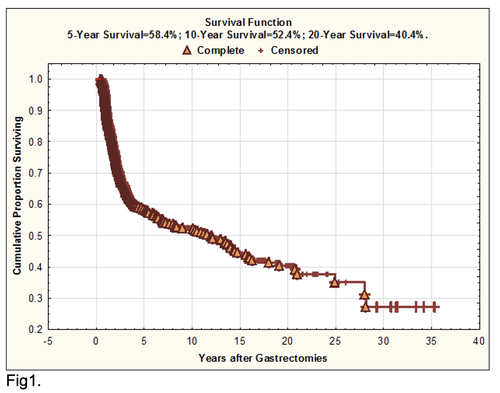
For the entire sample of 796 patients overall life span (mean±standard error) was 2130.8±2304.3 days (95% CI, 21196.4-2423.5; median=1089.5) and cumulative 5-year survival reached 58.4%, 10-year survival – 52.4%, 20-year survival – 40.4% (Fig.1). 316 GCP (life span=4316.1±2292.9 days) lived more than 5 years and 169 (life span=5919.5±2020 days) – more than 10 years without any features of GC progressing. 294 GCP (life span=640.6±347.1 days) died due to the cancer generalization within the first 5 years after complete gastrectomies.
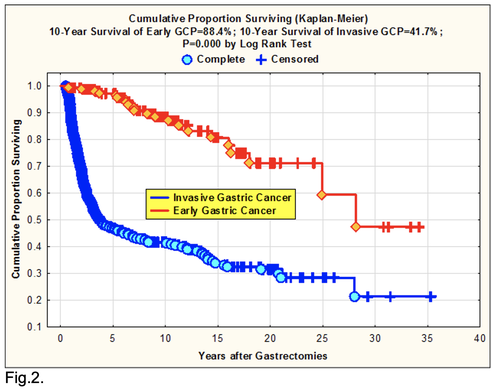
It is necessary to pay attention on the two very important prognostic phenomenons. First, 88.4 % 10-year survival for GCP with the early cancer as against 41.7% for the others GCP after gastrectomies (P=0.000 by log-rank test) (Fig.2). We understand as the early cancer the tumor up to 2 cm in diameter, witch invades submucosa without lymph node and distant metastases [10].
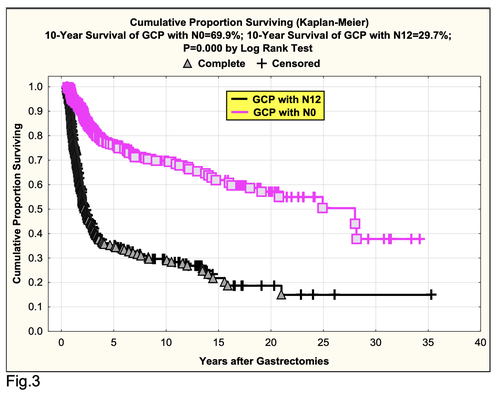
Second, good 10-year survival for GCP with N0 (69.9%) as compared with GCP with N1-2 (10-year survival was 29.7%) after radical procedures (P=0.000 by log-rank test) (Fig.3).
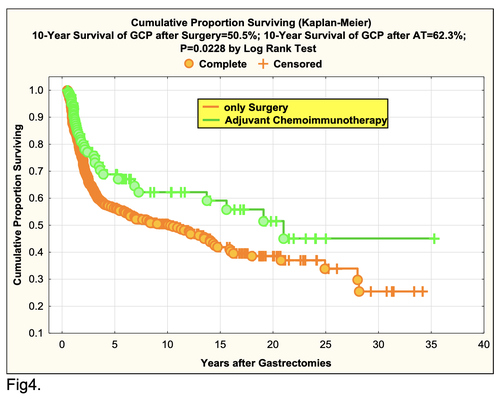
For the 173 GCP in adjuvant chemoimmunotherapy (AT) arm (group A) cumulative 10-year survival reached 62.3% vs. 50.5% (group B) (P=0.0228 by log-rank test) (Fig.4).
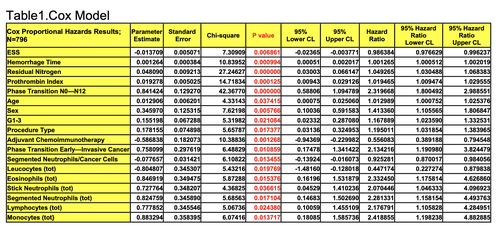
All clinicopathologic characteristics and treatment modalities were evaluated in traditional Cox multivariate prognostic factor analysis. In accordance with Cox regression model, the 17 variables significantly explained GCP 10-year survival with N0-2 (n=796) after complete gastrectomies: Cox modeling displayed that 10YS of GCP significantly depended on: phase transition (PT) early-invasive GC in terms of synergetics, PT N0—N12, cell ratio factors (ratio between cancer cells- CC and blood cells subpopulations), G1-3, AT, blood cell circuit, prothrombin index, hemorrhage time, residual nitrogen, age, sex, procedure type (P=0.000-0.039)(Table 1).
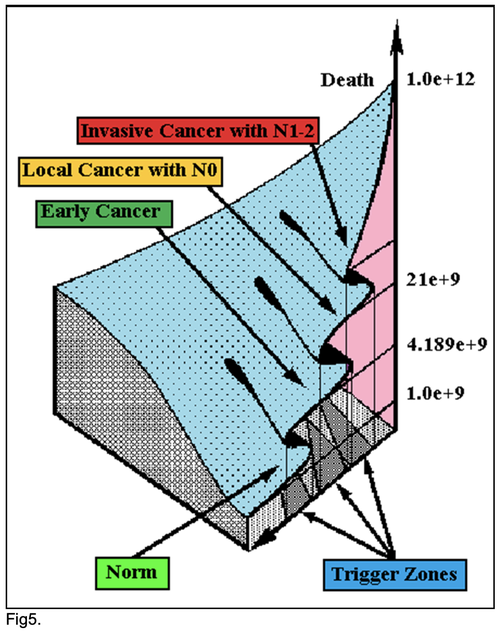
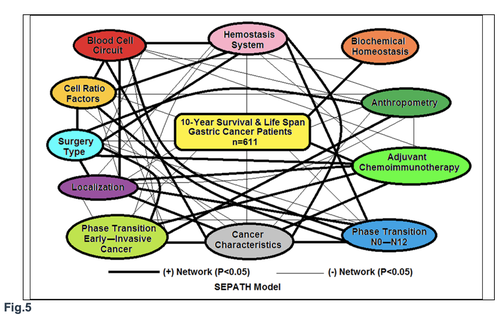
All of these differences and discrepancies were further investigated by structural equation modeling (SEPATH) as well as Monte Carlo simulation. For more exact analysis 185 patients were excluded from sample, which were alive less than 5 years after complete gastrectomies without relapse. From data, summarized in Fig. 5 it was revealed that the ten clusters significantly predicted 10-year survival and life span of GCP with N0-2 status (n=611): 1) PT early--invasive GC 2)PT N0—N12; 3) Cell Ratio Factors; 4)GC characteristics; 5) biochemical homeostasis; 6) hemostasis system; 7) surgery type; 8) adjuvant chemoimmunotherapy; 9) anthropometric data; 10) tumor localization.
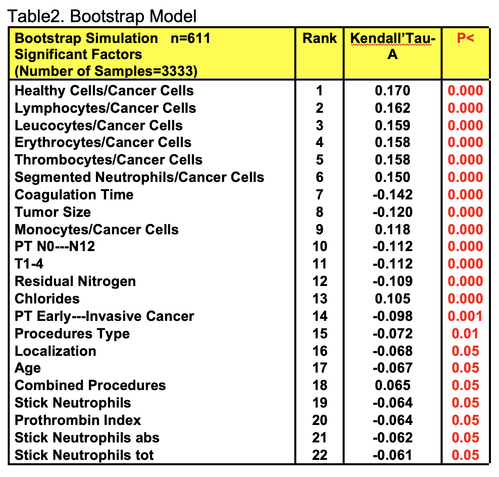
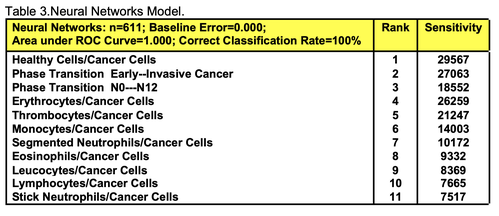
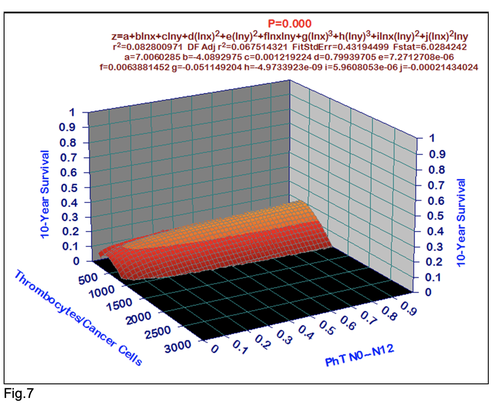
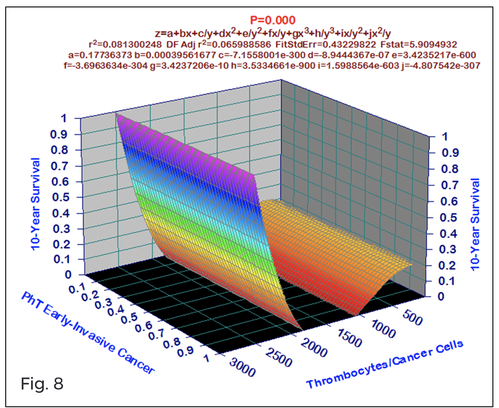
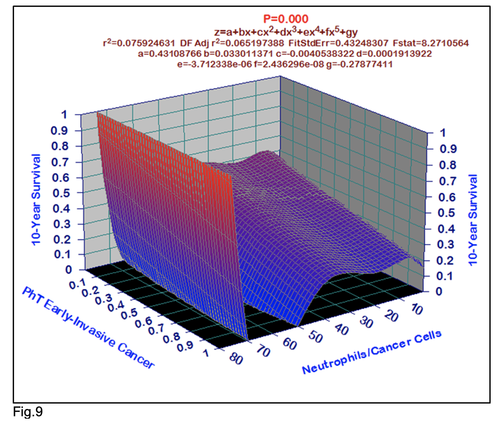
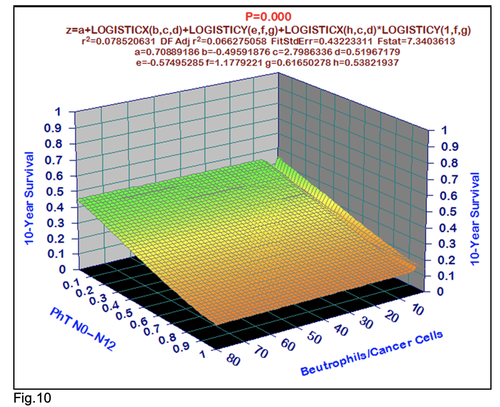
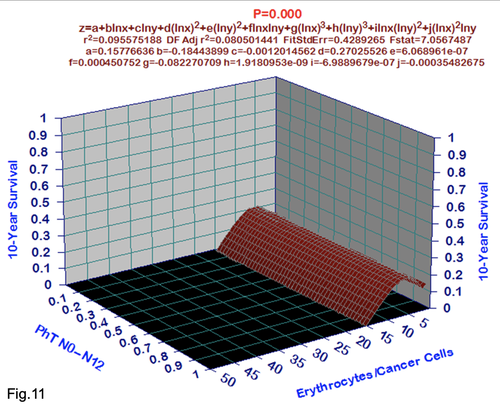
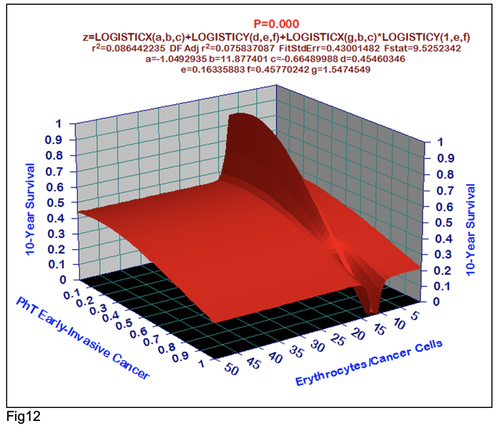
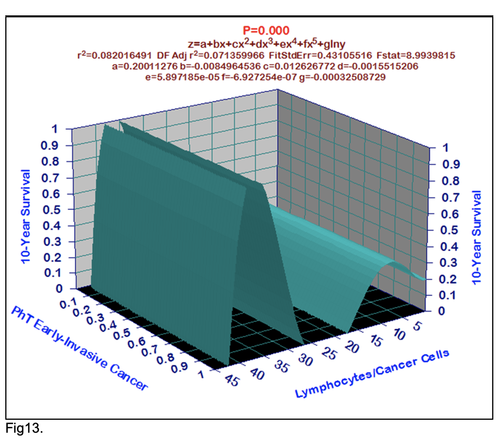
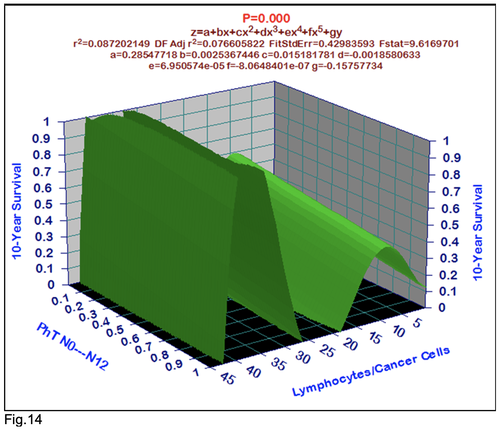
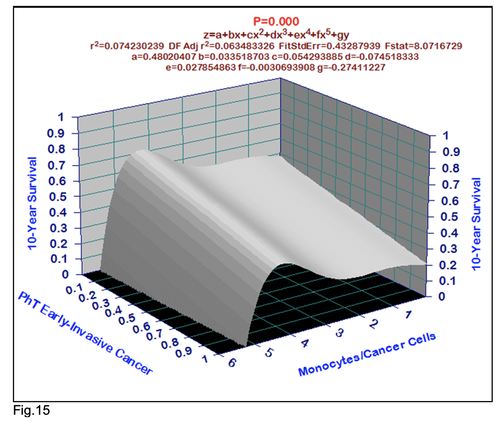
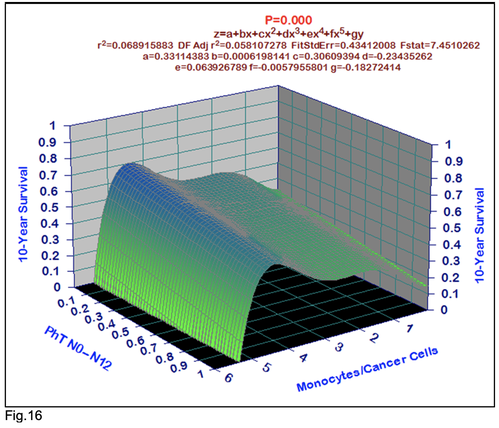
For comparative purposes, clinicopathological factors of GCP (n=611) were tested by neural networks computing (4-layer perceptron) (Table 3). Obviously, analyzed data provide significant information about GC prediction. High accuracy of classification (10-year survivors vs. losses) was achieved 100% (baseline error=0.000, area under ROC curve=1.0). In other words, it remains formally possible that at least 11 of these factors might predate neoplastic generalization: Healthy Cells/CC (rank=1), PT early---invasive GC (rank=2); PT N0---N12 (Rank=3); Erythrocytes/CC (4); Thrombocytes/CC (5); Monocytes/CC (6); Segmented Neutrophils (7); Eosinophils/CC (8); Leucocytes/CC (9); Lymphocytes/CC (10) Stick Neutrophils (11). Moreover, bootstrap simulation confirmed the paramount value of Cell Ratio Factors, PT N0---N12 and PT early---Invasive GC (Table 2). It is necessary to note a very important law: both transitions of the early cancer into the invasive cancer, as well as the cancer with N0 into the cancer with N1-N2, have the phase
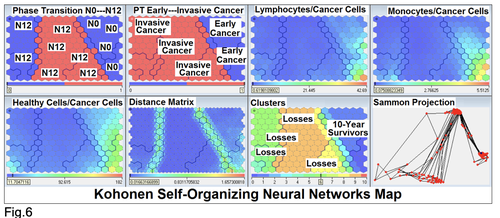
character. These results testify by mathematical and imitating modeling of system “GC—patient homeostasis” in terms of synergetics (Fig. 7-16). This also proves the first results received earlier in the work [10] (Fig.5). Presence of the two phase transitions is evidently shown on Kohonen self-organizing neural networks maps (Figure 6).
It is necessary to pay attention on the two very important prognostic phenomenons. First, 88.4 % 10-year survival for GCP with the early cancer as against 41.7% for the others GCP after gastrectomies (P=0.000 by log-rank test) (Fig.2). We understand as the early cancer the tumor up to 2 cm in diameter, witch invades submucosa without lymph node and distant metastases [10].
Second, good 10-year survival for GCP with N0 (69.9%) as compared with GCP with N1-2 (10-year survival was 29.7%) after radical procedures (P=0.000 by log-rank test) (Fig.3).
For the 173 GCP in adjuvant chemoimmunotherapy (AT) arm (group A) cumulative 10-year survival reached 62.3% vs. 50.5% (group B) (P=0.0228 by log-rank test) (Fig.4).
All clinicopathologic characteristics and treatment modalities were evaluated in traditional Cox multivariate prognostic factor analysis. In accordance with Cox regression model, the 17 variables significantly explained GCP 10-year survival with N0-2 (n=796) after complete gastrectomies: Cox modeling displayed that 10YS of GCP significantly depended on: phase transition (PT) early-invasive GC in terms of synergetics, PT N0—N12, cell ratio factors (ratio between cancer cells- CC and blood cells subpopulations), G1-3, AT, blood cell circuit, prothrombin index, hemorrhage time, residual nitrogen, age, sex, procedure type (P=0.000-0.039)(Table 1).
All of these differences and discrepancies were further investigated by structural equation modeling (SEPATH) as well as Monte Carlo simulation. For more exact analysis 185 patients were excluded from sample, which were alive less than 5 years after complete gastrectomies without relapse. From data, summarized in Fig. 5 it was revealed that the ten clusters significantly predicted 10-year survival and life span of GCP with N0-2 status (n=611): 1) PT early--invasive GC 2)PT N0—N12; 3) Cell Ratio Factors; 4)GC characteristics; 5) biochemical homeostasis; 6) hemostasis system; 7) surgery type; 8) adjuvant chemoimmunotherapy; 9) anthropometric data; 10) tumor localization.
For comparative purposes, clinicopathological factors of GCP (n=611) were tested by neural networks computing (4-layer perceptron) (Table 3). Obviously, analyzed data provide significant information about GC prediction. High accuracy of classification (10-year survivors vs. losses) was achieved 100% (baseline error=0.000, area under ROC curve=1.0). In other words, it remains formally possible that at least 11 of these factors might predate neoplastic generalization: Healthy Cells/CC (rank=1), PT early---invasive GC (rank=2); PT N0---N12 (Rank=3); Erythrocytes/CC (4); Thrombocytes/CC (5); Monocytes/CC (6); Segmented Neutrophils (7); Eosinophils/CC (8); Leucocytes/CC (9); Lymphocytes/CC (10) Stick Neutrophils (11). Moreover, bootstrap simulation confirmed the paramount value of Cell Ratio Factors, PT N0---N12 and PT early---Invasive GC (Table 2). It is necessary to note a very important law: both transitions of the early cancer into the invasive cancer, as well as the cancer with N0 into the cancer with N1-N2, have the phase
character. These results testify by mathematical and imitating modeling of system “GC—patient homeostasis” in terms of synergetics (Fig. 7-16). This also proves the first results received earlier in the work [10] (Fig.5). Presence of the two phase transitions is evidently shown on Kohonen self-organizing neural networks maps (Figure 6).
FigureS 5-16
Click on each figure to enlarge
DISCUSSION
Central goal of the present research was to estimate the efficiency of adjuvant chemoimmunotherapy after complete gastrectomies. The importance must be stressed of using complex system analysis, artificial intelligence (neural networks computing), statistical methods and simulations in combination, because the different approaches yield complementary pieces of prognostic information [6, 25].
Although there is no consensus on adjuvant treatment followed by radical procedures two of the most commonly employed strategies are surgery alone and adjuvant chemotherapy with or without immunotherapy.
Actually surgical removal of tumor and its metastases remains basic management of this very aggressive cancer giving the real chance for recovery in spite of quite intensive researches developed during the last 30 years in terms of chemotherapy and immunotherapy [16,17]. Unfortunately, the effectiveness of complete gastrectomies (total, distal and proximal) has already reached its limit and leaves much to be desired: the average real 5-year survival rate of radically operated GCP even after combined and extensive procedures is 30-35% and practically is not improved during the past 50 years, as the great majority of patients has already GC with stage II-III [5,18].
In the last 10-20 years a number of new drugs have been shown to have good activity against GC, including mitomycin C, UFT, epirubicin, etoposide, cisplatin, doxetacel, irinotecan, etc. [19,20-22]. On the other hand new immunomodulators, checkpoint inhibitors, new adoptive immunotherapeutic modalities with lymphokine-activated killer cells, tumor-infiltrating lymphocytes and high-dose interleukins have been developed and antitumor effect have been successfully demonstrated in advanced malignancies, including GC [4,7,23-25].
Theoretically chemoimmunotherapy is most effective when used in patients with a relatively low residual malignant cell population (approximately 1 billion cancer cells per patient) in terms of hidden micrometastases [6]. This is typical clinical situation at GCP with stage II-III after complete gastrectomies (R0). Present research only confirmed this axiom.
In summary, when adjuvant chemoimmunotherapy is applied to complete gastrectomies for GC, the following benefits should be considered: 1) possibility of total elimination of residual hidden micrometastases; 2) surgery and chemotherapy can result immunosuppressive state, which can be improved by immunotherapy; 3) radical operated GCP with stage II-III are thought to be potentially good candidates for adjuvant chemoimmunotherapy as the majority of these patients would be expected to have GC progressing.
Further investigations will be required to determine efficiency, compatibility and tolerance of new drugs and immunomodulators, checkpoint inhibitors after gastrectomies. The results of the present research will offer guidance for the design of future studies.
Although there is no consensus on adjuvant treatment followed by radical procedures two of the most commonly employed strategies are surgery alone and adjuvant chemotherapy with or without immunotherapy.
Actually surgical removal of tumor and its metastases remains basic management of this very aggressive cancer giving the real chance for recovery in spite of quite intensive researches developed during the last 30 years in terms of chemotherapy and immunotherapy [16,17]. Unfortunately, the effectiveness of complete gastrectomies (total, distal and proximal) has already reached its limit and leaves much to be desired: the average real 5-year survival rate of radically operated GCP even after combined and extensive procedures is 30-35% and practically is not improved during the past 50 years, as the great majority of patients has already GC with stage II-III [5,18].
In the last 10-20 years a number of new drugs have been shown to have good activity against GC, including mitomycin C, UFT, epirubicin, etoposide, cisplatin, doxetacel, irinotecan, etc. [19,20-22]. On the other hand new immunomodulators, checkpoint inhibitors, new adoptive immunotherapeutic modalities with lymphokine-activated killer cells, tumor-infiltrating lymphocytes and high-dose interleukins have been developed and antitumor effect have been successfully demonstrated in advanced malignancies, including GC [4,7,23-25].
Theoretically chemoimmunotherapy is most effective when used in patients with a relatively low residual malignant cell population (approximately 1 billion cancer cells per patient) in terms of hidden micrometastases [6]. This is typical clinical situation at GCP with stage II-III after complete gastrectomies (R0). Present research only confirmed this axiom.
In summary, when adjuvant chemoimmunotherapy is applied to complete gastrectomies for GC, the following benefits should be considered: 1) possibility of total elimination of residual hidden micrometastases; 2) surgery and chemotherapy can result immunosuppressive state, which can be improved by immunotherapy; 3) radical operated GCP with stage II-III are thought to be potentially good candidates for adjuvant chemoimmunotherapy as the majority of these patients would be expected to have GC progressing.
Further investigations will be required to determine efficiency, compatibility and tolerance of new drugs and immunomodulators, checkpoint inhibitors after gastrectomies. The results of the present research will offer guidance for the design of future studies.
REFERENCES
1. Janunger K.G., Hafstrom L., Nygren R., Glimelius B. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001; 40(2-3): 309-326.
2. Morant R. Neoadjuvant and adjuvant chemotherapy of locally advanced stomach cancer. Onkologie. 2001; 24(2): 116-121.
3. Kshivets O. Adjuvant chemo- and immunotherapy of gastric cancer. Experimental Oncology. Kiev: 1991: 24pp.(VINITI 06.06.91.-N2383-B91).
4. Koji Kono, Shotaro Nakajima, Kosaku Mimura Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer, 2020 Jul;23(4):565-578.
5. Kim J.P. Surgical results in gastric cancer. Semin Surg Oncol. 1999; 17(2): 132-138.
6. Kshivets O. Expert system in diagnosis and prognosis of malignant neoplasms. Dissertation for Sc.D., Tomsk, 1995: 639pp.
7. Gochi A., Orita K., Fuchimoto S. et al. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br J Cancer. 2001; 84(4): 443-451.
8. Mavroudis D., Kourousis C., Androulakis N. et al. Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): a phase II trial. Am J Clin Oncol. 2000; 23(4): 341-344.
9. Kim J.P., Yu H.J., Lee J.H. Results of immunochemo-surgery for gastric carcinoma. Hepatogastroenterology. 2001; 48(41): 1227-1230.
10. Morozow V.G., Chavinson V.C. Isolation, refinement and identification of immunomodulated polypeptide from calf and human thymus. Biochemistry (Russia) 1981;9:1652-59.
11. Odom-Maryon T. Biostatistical methods in oncology. Cancer management: A multidisciplinary approach. 1st ed. Huntington, NY: PRP Inc., 1996: 788-802.
12. Mirkin B.G. A sequential fitting procedure for linear data analysis models. J Classification 1990; 7: 167-196.
13. Joreskog K.G., Sorbom D. Recent development in structural equation modeling. J Marketing Research 1982; 19: 404-416.
14. Bostwick D.G., Burke H.B. Prediction of individual patient outcome in cancer: comparison of artificial neural networks and Kaplan-Meier methods. Cancer. 2001; 91(8):1643-1646.
15. Husmeier D. The Bayesian evidence scheme for regularizing probability-density estimating neural networks. Neural Comput. 2000; 12(11): 2685-2717.
16. Fukushima M. Adjuvant therapy of gastric cancer: the Japanese experience. Semin Oncol. 1996; 23(3): 369-378.
17. Yao J.C., Shimada K., Ajani J.A. Adjuvant therapy for gastric carcinoma: closing out the century. Oncology (Huntingt). 1999; 13(11): 1485-1502.
18. Kshivets O. Surgical and combined treatment of gastric cancer. Questions of Oncology 1991; 7-8: 788-795.
19. Schuhmacher C.P., Fink U., Becker K. et al. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001; 91(5): 918-927.
20. Mari E., Floriani I., Tinazzi A., et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomized trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol. 2000;11(7): 837-843.
21. Cirera L., Balil A., Batiste-Alentorn E. et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer.J Clin Oncol. 1999; 17(12): 3810-3815.
22. Shimada K., Ajani J.A. Adjuvant therapy for gastric carcinoma patients in the past 15 years: A review of western and oriental trials. Cancer. 1999; 86(9): 1657-1668.
23. Yano T., Sugio K., Yamazaki K., et al. Postoperative adjuvant adoptive immunotherapy with lymph none-LAK cells and IL-2 for pathologic stage I non-small cell lung cancer. Lung Cancer 1999; 26: 143-8.
24. Kshivets O. Immune cell and humoral circuit in prediction of non-small cell lung cancer patients survival after complete resections. Journal of Tumor Marker Oncology 2001; 16(2): 161-174.
25. Kshivets O. Esophageal cancer: Optimization of management. The Open Cardiovascular and Thoracic Surgery Journal, 2008, 1, 1-11.
1. Janunger K.G., Hafstrom L., Nygren R., Glimelius B. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001; 40(2-3): 309-326.
2. Morant R. Neoadjuvant and adjuvant chemotherapy of locally advanced stomach cancer. Onkologie. 2001; 24(2): 116-121.
3. Kshivets O. Adjuvant chemo- and immunotherapy of gastric cancer. Experimental Oncology. Kiev: 1991: 24pp.(VINITI 06.06.91.-N2383-B91).
4. Koji Kono, Shotaro Nakajima, Kosaku Mimura Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer, 2020 Jul;23(4):565-578.
5. Kim J.P. Surgical results in gastric cancer. Semin Surg Oncol. 1999; 17(2): 132-138.
6. Kshivets O. Expert system in diagnosis and prognosis of malignant neoplasms. Dissertation for Sc.D., Tomsk, 1995: 639pp.
7. Gochi A., Orita K., Fuchimoto S. et al. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br J Cancer. 2001; 84(4): 443-451.
8. Mavroudis D., Kourousis C., Androulakis N. et al. Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): a phase II trial. Am J Clin Oncol. 2000; 23(4): 341-344.
9. Kim J.P., Yu H.J., Lee J.H. Results of immunochemo-surgery for gastric carcinoma. Hepatogastroenterology. 2001; 48(41): 1227-1230.
10. Morozow V.G., Chavinson V.C. Isolation, refinement and identification of immunomodulated polypeptide from calf and human thymus. Biochemistry (Russia) 1981;9:1652-59.
11. Odom-Maryon T. Biostatistical methods in oncology. Cancer management: A multidisciplinary approach. 1st ed. Huntington, NY: PRP Inc., 1996: 788-802.
12. Mirkin B.G. A sequential fitting procedure for linear data analysis models. J Classification 1990; 7: 167-196.
13. Joreskog K.G., Sorbom D. Recent development in structural equation modeling. J Marketing Research 1982; 19: 404-416.
14. Bostwick D.G., Burke H.B. Prediction of individual patient outcome in cancer: comparison of artificial neural networks and Kaplan-Meier methods. Cancer. 2001; 91(8):1643-1646.
15. Husmeier D. The Bayesian evidence scheme for regularizing probability-density estimating neural networks. Neural Comput. 2000; 12(11): 2685-2717.
16. Fukushima M. Adjuvant therapy of gastric cancer: the Japanese experience. Semin Oncol. 1996; 23(3): 369-378.
17. Yao J.C., Shimada K., Ajani J.A. Adjuvant therapy for gastric carcinoma: closing out the century. Oncology (Huntingt). 1999; 13(11): 1485-1502.
18. Kshivets O. Surgical and combined treatment of gastric cancer. Questions of Oncology 1991; 7-8: 788-795.
19. Schuhmacher C.P., Fink U., Becker K. et al. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001; 91(5): 918-927.
20. Mari E., Floriani I., Tinazzi A., et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomized trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol. 2000;11(7): 837-843.
21. Cirera L., Balil A., Batiste-Alentorn E. et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer.J Clin Oncol. 1999; 17(12): 3810-3815.
22. Shimada K., Ajani J.A. Adjuvant therapy for gastric carcinoma patients in the past 15 years: A review of western and oriental trials. Cancer. 1999; 86(9): 1657-1668.
23. Yano T., Sugio K., Yamazaki K., et al. Postoperative adjuvant adoptive immunotherapy with lymph none-LAK cells and IL-2 for pathologic stage I non-small cell lung cancer. Lung Cancer 1999; 26: 143-8.
24. Kshivets O. Immune cell and humoral circuit in prediction of non-small cell lung cancer patients survival after complete resections. Journal of Tumor Marker Oncology 2001; 16(2): 161-174.
25. Kshivets O. Esophageal cancer: Optimization of management. The Open Cardiovascular and Thoracic Surgery Journal, 2008, 1, 1-11.
The data in this poster was presented at World Congress of Gastrointestinal Cancer 2021. Published with permission from the Copyright owner.
